|
Section
0401 |
|
|
February
2016 (Volume
66 - Issue 2) |
|
Our
objective: To increase awareness,
interest, and involvement in Section activities and quality-related subjects. Visit our web site at |
| 10 - The Interview Corner | 16 - Unemployed Member Dues | |
| 17 - Feedback/Advertising Rates | ||
1.
Next
Event
Date Wednesday, February 24, 2016 Time 6:00 PM Place Modern
GMP Science and Risk Based Approach for the 21st Century
 Mr. Ady Sadek President proGamma Science Corporation **************************************
Please join us on Wednesday, February 24th to hear our guest speaker present the topic of Modern GMP Science and Risk Based Approach for the 21st Century.
|
2.
Ad
/
Publicité
|
||||||||||||||||||||
|
ABOUT THE PRESENTATION Modern Good Manufacturing Practice in the pharmaceutical industry is moving from reacting to problems to preventing problems through assessing risks to the manufacturing process. Pharmaceutical and biopharmaceutical manufacturing is changing and evolving very rapidly. Many companies are involved in improving and enhancing decision making to gain more efficiencies and therefore competitiveness. The use of GMP aligned with Risk Management provides the industry an opportunity to “enhance” the decision making process within the current regulatory context as per HPFB, WHO, FDA and EMA. Modern regulatory inspections require attention to various details of which many companies in the USA, Canada and Europe miss and fall into non-compliance. This presentation will explore the concepts, techniques and regulatory requirements, basically the how and why, which result in excellent understanding as well as commitment of personnel to the training and solid understanding of modern cGMPs. ABOUT OUR SPEAKER Before joining proGamma, Ady Sadek spent 25 years in the pharmaceutical industry with BioResearch, Schering-Plough and Novartis Pharmaceuticals, holding positions such as Manager of Analytical & Development Labs, Director of Quality and Director of Quality Audit. He holds two master degrees in organic and analytical chemistry, and is an ISO 9000 lead assessor. Mr. Sadek's vast experience in GMP compliance, process design and validation started in 1981, when developing new formulations for FDA submissions. He has been involved in many Plant GMP training and audits, process and computer systems validations, and his experience extends from formulation development to scale up commercial batches and technology transfer. He has consulted and lectured in Quality by Design, GMP, Risk Management, Process Validation, CSV and modern quality systems throughout Canada, USA and Europe to several multinationals. *****************************************
Bring
your business cards and be ready to
network. To
register for any event or for more information on events please
contact:
Dr. David Tozer  E-mail: event@asqmontreal.qc.ca 3. Upcoming Section Events
Don't miss the
following upcoming events of the Montreal Section 401 Chapter! 4. The Editor's Corner
It is one of the great banes of Quality professionals, especially those who wear the supplier hat: customer requirements that go over and above the normal quality standard requirements, as spelled out in ISO9001, AS9100, ISO14001, etc. Many have surely had a customer say the following: “We know your system is compliant to (insert standard name here) and that you have been certified since (insert year here). However, our specific requirements on (insert QMS process here) require you to do (insert additional requirement(s) here).” I encountered one of these situations recently when one of my company’s customers wanted to assure that we had a process in place to track the movement of customer supplied property (CSP) in our facility. This is not a requirement in the current revision of AS9100 (7.5.4), but as often is the case, you want to keep the customer happy and, of course, be compliant to their requirements. The funny thing about this customer requirement though was that it does not even seem to be an actual requirement of theirs! The issue of tracking CSP movement was a question on a customer questionnaire that was filled out by us, where we responded that we currently do not do it. As a result of that answer, and other shortcomings identified on the completed questionnaire, we put together an action plan to address all the issues. Pretty standard fair. However, when I asked to see the actual customer requirement on CSP tracking (in order to properly upgrade our own process), I was provided with 2 documents that I was told contained the requirement. After careful reading, I concluded that no such requirement existed! It talked about recording the “location” of CSP in a supplier’s facility, but that is as close as it got. Recording the location of where a specific CSP is located/stored is something we already do. “Location” however is not the same as “Movement”. In truth though, I actually have nothing against tracking the movement of CSP in our facility, and potentially having it be a best practice. The thing that got to me was being told that it was a customer requirement, yet not being given any evidence to prove that it was. As I noted at the beginning, additional customer requirements can be a bane, but if they are truly required, then let them at least be properly documented. *******************************************************
Any feedback? Click on the link in the bottom right corner of this section and let me know. Thanks. |
|||||||||||||||||||||
5.
A Word
from
your Section Chair
As we know, the quality field has a very rich toolbox. Some are old, some not so old and brand new ones – or slight modifications of old tools – are added every year. This is no surprise since quality is an evolving field. If you browse any issue of Quality Progress, you will find that at least one new idea is presented. A variety of sciences have contributed to the field of quality: metrology, project management, and statistics, to name a few. Sometimes the introduction of a new tool or approach has the negative effect of reinforcing the belief that older methods are obsolete. It would be like advocating that with real time data acquisition devices and algorithmic process control, statistical process control is no longer needed. People who are very knowledgeable about process control know that this is false and actually, some processes could benefit of a control scheme based on both methodologies. It is clear to me that statistics is a core science in quality, and actually most of the basic quality tools have their roots in statistical science. The majority of the ASQ certifications’ body of knowledge include statistics. As a “new shiny object” is introduced to quality professionals, our enthusiasm to try and possibly adopt it should be accompanied with a desire to find where it would best fit our current toolbox, without throwing away the proven tools already in it. |
|||||||||||||||||||||
6.
Had
You Come
to the
Last Event
By Gordon
Ayotte, ASQ Director, CQE, CQM, CSSBB
 Had you come . . . Networking Event Had
you come to the ASQ Networking event of January 27, 2016 you would have
taken part in a very dynamic evening held in a wonderful venue at the
Essence Restaurant downtown Montreal, in addition to the delicious and
exotic hors-oeuvre served during the event.
You would have also benefited form the interaction and feedback from the large number of participants, as well as taken part in a very interesting ice breaker exercise. Everyone got to exchange and know each other in a professional and social context, and hear feedback from people with different backgrounds on why they come to ASQ events, what they wish they knew at the beginning of their career, and what are some of the challenges they now face. 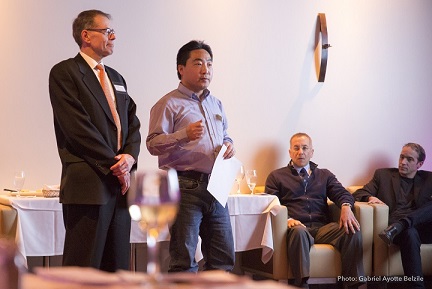 In addition, you would have benefited from the two kiosks: 1) Certification, which was set up to answer questions and promote the benefits of having an ASQ Certification; and, 2) Social Media, on how it can help keep someone current in the Quality field and help in career advancement. The topics of last year's events, as well as the upcoming topics for this year, were also discussed.  Please come and join us at our next event which will be held at the Sheraton Hotel at Trudeau Airport. |
|||||||||||||||||||||
7. Voice Of The
Customer
|
|||||||||||||||||||||
8.
Welcome
to our New Members
JANUARY
2016
Salma Michel Chahine Sergio Garon Jacques A. Grenieri Yannis Guillet Lei Guo Kesara R. Jayasuriya Jobin John Stuart Kozlick Sailakshmi Rajagopal Veronique Tokateloff |
|||||||||||||||||||||
9.
Organization
Members
ASQ Montreal Section thanks our Organization Members: |
|||||||||||||||||||||
10. The Interview Corner
We have no interview for you this month. Please check back in March 2016! 11.
Other
ASQ
Events
|
|||||||||||||||||||||
12. ASQ NewsOrganizational
Structure Update 2016
Lean and Six Sigma Conference
|
|||||||||||||||||||||
13.
ASQ
Montreal Section Education Program 2016
By
Dr. David
Tozer,
Ph.D., ASQ CQE and SSBB, Education & Audit Chair Having ASQ certification gives you an edge in the market and can significantly increase your income. ASQ Certification often leads to higher paying employment. The money invested in education and certification increases chances of finding employment quickly in the down sizing environment we live in. People who take the section sponsored refresher courses, and spend at least twice as much time as spent in the classroom on self study, have an 80%, or better, chance of passing the examination on the first attempt. Certified Quality Engineer Topics include: quality concepts, cost of quality, human resources, team formation and group dynamics, inspection, metrology, sampling, reliability, quality standards, quality audit, statistics, design of experiments, process improvement, liability, and modern management methods for improving quality. Certified Six Sigma Black Belt Topics include: quality concepts, cost of quality, enterprise wide deployment, business process management, project management, team formation and group dynamics, define, measure, analyze, improve, control, lean enterprise, statistics, design of experiments, and design for six sigma. Certified Six Sigma Green Belt Topics include: quality concepts, cost of quality, enterprise wide deployment, business process management, project management, team formation and group dynamics, define, measure, analyze, improve, control, and statistics. Certified Manager of Quality/Organizational Excellence Topics include: quality concepts, quality planning, customer focus, quality standards, project management, cost of quality, team formation and group dynamics, human resources and improvement.
Certified
Quality Auditor Topics
include: quality concepts,
team
formation and group dynamics, management responsibility, audit
objectives, audit preparation, audit conduct, audit reporting,
sampling, and basic statistics.
Certified Quality Inspector Topics include: quality concepts, team formation and group dynamics, geometry, metrology, reading drawings, mechanical processes, statistical process control, inspection, and sampling. Calendar and Registration Form Questions?
In house
courses, etc.: Dr. David Tozer:
(514) 694-2830,
|
|||||||||||||||||||||
14.
Executive
Committee Meetings & Officers
Section Executive
Committee
(Leadership Team) Meetings are held at different locations, starting at
6 PM. The next regular meeting is tentatively scheduled for: March
2, 2016 Consult the List of Your Executive
for
2016 here |
|||||||||||||||||||||
15. RecertificationIs
Your
Recertification Due?
Look at your wallet card to see when your present certification is due to expire. If it says December 31, 2015 you should contact ASQ by phone at 1-800-248-1946 and explain your situation to them because your certification has lapsed and you are no longer certified. If your wallet card states June 2016 you have until December 2016 to get your journal to me. Why so long? Because you have your expiration date of June 20, 2016 plus or minus six months. Maybe you’ve decided not to recertify because (a) you are unemployed, (b) no longer in the quality field or perhaps, (c ) your employer no longer will pay for it? Think about this, your certification belongs to you and no one else. Your name is on it and no one else’s. It is portable and you can bring the recognition to your next company. Remember how hard you had to study for it? If you let it lapse you must rewrite the exam. Do you know where you will be employed in a year or so? Well congratulations if you do because most of us don’t and it could come in handy then, it sure won’t hinder you to retain it. The cost at $69 USD to renew one certification is much less than it would to rewrite. If you are unemployed then contact ASQ directly at 1-800-248-1946. Ask for “Recertification” then explain your unemployment situation to them. You may be able to have your due date extended. But at $69.00 that is not really that much if it will help land your next job? If you are a member of Section 0401, Montreal then contact me (Norman) at certification@asqmontreal.qc.ca to find out where to send your journal. If you are NOT a member of section 401 then contact ASQ directly at 1-800-248-1946. Please DO NOT SEND your journal by e-mail. Use Canada Post or equivalent. 16.
Unemployed Member Dues
Unemployed ASQ members receive a discount on their membership dues based on consecutive years of membership.
Eligibility Criteria
Benefits
NOTE: The following links require that you be logged into your account before you try to activate them. Download the ASQ Unemployment Program
Application
PDF (105 KB) |
|||||||||||||||||||||
17.
Feedback
Please send us your comments about the ASQ Montreal Section 0401 E-Newsletter (topics, layout, length, etc.). Do you want to contribute an article (English or French) or a good idea? Contact us by e-mail. |
|
||||||||||||||||||||
Pass
it on
We invite you to forward this Newsletter to friends and colleagues who may be interested. |
|||||||||||||||||||||
|
The
ASQ
Montreal
Section 0401 Newsletter is prepared by and published for its members. How to
Opt Out. This
e-mail
is being sent in the course of normal Montreal Section
business
to the e-mail address of record. We are not responsible for forwarded
e-mails. If you no longer wish to receive e-mail communications from
the Montreal Section (your section) of ASQ, please visit your ASQ
account to unsubscribe or
reply to this message, indicating Opt
Out in the
e-mail body
and in the title. |
|||||||||||||||||||||




